We have seen from the previous texts that we are surrounded by small amounts of radioactivity “everywhere”. Often we use the terms NORM and TENORM for this activity.
NORM denotes "Naturally Occurring Radioactive Material" and TENORM stands for Technologically-Enhanced Naturally Occurring Radioactive Material."
NORM and TENORM are important and complex issues that involve science, politics, business, and the public.
- NORM is everywhere; we are exposed to it every day. Natural radiation has been with us since the “Big Bang”.
But some industrial practices involving natural resources concentrate these radionuclides to a degree that they may pose risk to humans and the environment if they are not controlled.
TENORM is found in many waste streams; for example, scrap metal, sludges, slags, fluids, and is being discovered in industries traditionally not thought of as affected by radionuclide contamination like for instance the petroleum industry.
The majority of radionuclides in TENORM are found in the uranium and thorium decay chains. The decay products of Radon are the largest source of natural radioactivity we are exposed to.
Radium and radon are the principal radionuclides used to measure NORM and TENORM in the environment.
| Table 1: Typical concentration of NORM radionuclides in some common rocks |
||||||||
|---|---|---|---|---|---|---|---|---|
| Rock Type |
Potassium-40 |
Rubidium-87 |
Thorium-232 |
Uranium-239 |
||||
| Igneous Rocks |
Percent total Potassium |
Bq/Kg |
ppm totalt rubidium |
Bq/kg |
ppm |
Bq/kg |
ppm |
Bq/kg |
| Basalt (crustal average) |
0.8 |
300 |
40 |
30 |
3.4 |
10-15 |
0.5-1 |
7-10 |
| Mafic |
0.3-1.1 |
70-400 |
10-50 |
1-40 |
1.6,2,7 |
7,10 |
0.5,0.9 |
7,10 |
| Salic |
4.5 |
1,100-1,500 |
170-200 |
150-180 |
16,20 |
60,80 |
3.9,4.7 |
40,60 |
| Granite (Crustal average) |
>4 |
>1000 |
170-200 |
150-180 |
17 |
70 |
3 |
40 |
| Sedimentary Rocks |
||||||||
| Shale sandstone |
2.7 |
800 |
120 |
110 |
12 |
50 |
3.7 |
40 |
| Clean Quartz |
<1 |
<300 |
<40 |
<40 |
<2 |
<8 |
<1 |
<10 |
| Dirty Quartz |
2? |
400? |
90? |
80? |
3-6? |
10-25? |
2-3? |
40? |
| Arkose |
2-3 |
600-900 |
80-120 |
80 |
2? |
<8 |
1-2? |
10-25? |
| Beach Sands (Unconsolidated) |
<1 |
<300? |
<40? |
<40? |
6 |
25 |
3 |
40 |
| Carbonated Rocks |
0.3 |
70 |
10 |
8 |
2 |
8 |
2 |
25 |
| Continental Upper Crust |
||||||||
| Average |
2.8 |
850 |
122 |
100 |
10.7 |
44 |
2.8 |
36 |
| Soils |
1.5 |
400 |
65 |
50 |
9 |
37 |
1.8 |
66 |
Everyday radioactivity
Our food contains small amounts of various radionuclides, see table below.
| Table 2: Natural Radioactivity in Food |
|||
|---|---|---|---|
| Food |
40K Bq/kg |
226Ra Bq/kg |
|
| Banana |
130 |
0.04 |
|
| Brazil Nuts |
210 |
40-260 |
|
| Carrot |
125 |
0.02-0.07 |
|
| White Potatoes |
125 |
0.04-0.1 |
|
| Beer |
25 |
--- |
|
| Red Meat |
110 |
0.02 |
|
| Lima Bean raw |
170 |
0.07-0.2 |
|
| Drinking water |
0-0.006 |
||
We will limit examples here to two radionuclides that most people have contact with on a daily basis, namely 40K and 241Am.
40K is, as previously mentioned, a primordial radionuclide with a half-life of 1.26•109 years. Hence, it is naturally occurring in all natural constituents containing the chemical element potassium.
Its average natural abundance is rather low,- only 0.0117%. It decays by emitting a relatively high-energy beta particle of 1.33 MeV with an associated gamma ray of 1462 keV.
 |
|
|---|---|
| Fig 1: Slightly radioactive salt, not so radioactive pepper |
Look around you in the laboratory and try to spot potassium-containing chemicals: Do you see common chemicals as KCl or KOH for instance? They are radioactive! Do you use Seltin (so-called health salt) in your food? It contains nearly 50 % KCl and is radioactive. There is a laboratory exercise which looks closer at this.
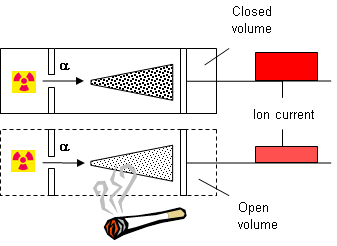
241Am is not really a NORM radionuclide in the common meaning of the word although it is produced as a waste product in the nuclear energy industry. However, most of us have a close contact with this radionuclide because it is used in so-called ionic smoke detectors. 241Am has a half-life of 432 years and decays by emitting alpha particles followed by a soft gamma ray of 60 keV. The working principle of one type of a smoke detector is sketched to the left. One sealed chamber contains a 241Am source. It irradiates the air in the chamber with alpha particles and create ions. The ions set up a current of a certain size between electrodes. A parallel chamber is perforated to let in ambient air. This also contains an identical source, and the ion current is measured. The size of the two currents shall be within a tolerance limit. When the atmosphere changes in the perforated chamber, for instance by intruding smoke particles from a fire, the ion current is affected (decreased). When the decrease is outside the tolerance limit, an alarm is set off, see below for an illustration.
 |
|---|
| Fig 2 How a smoke detector works. |
NORM and TENORM in the petroleum industry
Radioactivity associated with the recovery of petroleum products was released for the first time as early as in 1904 in connection with oil and gas production in Canada. This phenomenon did not catch much attention until the beginning of 1981 when central parts of the North Sea also experienced the same phenomenon. A precipitate, scale, was formed upon water breakthrough in production wells. This precipitate was radioactive. Activity levels in this scale were reported to vary between 0 and 15.000 Bq/g. In the following years similar reports came from other parts of the world as well.
Radioactive contaminants of natural gas have been known since early in this century. However, it was not until 1971 that this radioactivity, identified as Radon-222, hereafter referred to with its correct technical notion 222Rn, was found to concentrate in the lighter natural gas liquids during processing and could present a serious health hazard to industry personnel, particularly maintenance employees.
Due to frequently experienced high activity concentrations (Becquerel per unit weight or volume, for instance Bq/g or Bq/L), radioactivity in scale started to become an environmental concern both with respect to the working environment for the oil and gas workers and with respect to treatment and storage. Radioactivity associated with the production of oil and gas has by now been established as an important discussion subject in the oil and gas industry and a number of reports on its origin, occurrence, composition, distribution, treatment, dissolution, monitoring, storage and even avoidance have been published.
Radioactive scale is commonly termed LSA (Low Specific Activity) scale in Europe and NORM (Naturally Occurring Radioactive Material) scale in USA. One may also encounter the term TENORM (Technologically Enhanced Naturally Occurring Material). In the present text we will use the Norwegian acronym LRA (lav-radioaktive avleiringer).